Neutrons within a nucleus
The properties of an atomic nucleus depend on both the number of protons and on neutron numbers. The number of protons (the atomic number) determines the chemical properties, i.e. which element it is. The number of neutrons affects the stability of the nucleus.
With their positive charge, the protons within the nucleus are repelled by the long-range electromagnetic force, this repulsion between a group of protons would, by itself, make the nucleus unstable. The much stronger, but short-range, nuclear force which applies to both protons and neutrons, binds the nucleons closely together, so neutrons provide the stability of nuclei.
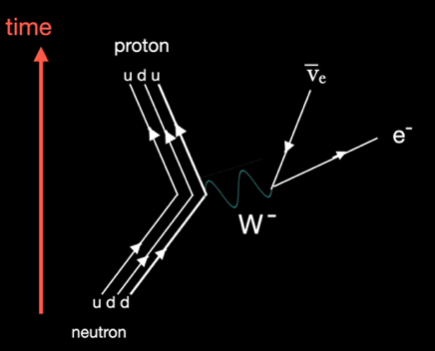
Neutrons within in a nucleus are mostly stable but can be unstable, depending on the particular nuclide. If unstable then neutron decay results in beta emission. Neutrons decay to protons governed by the weak force, requiring the emission of an electron and an antineutrino.
Free neutrons
A free neutron is unstable, it will decay to a proton emitting an electron and an antineutrino with a mean lifetime of about 15 minutes. This radioactive decay, is also beta decay, the neutron decays in the same way as a neutron within a nucleus. Decay is is possible because the mass of the neutron is slightly greater than that of the proton, that small change in mass converts to kinetic energy. A free proton is stable.
The decay process
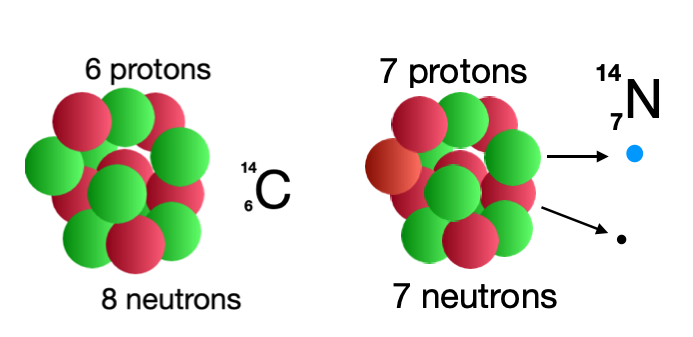
Either within a nucleus or as an individual free particle, when a neutron decays it does so in the same way, becoming a stable proton and emitting an electron and an antineutrino. If the neutron is within a nucleus, the nature of that isotope changes. For example carbon 14 is a radioactive isotope containing 8 neutrons and 6 protons within the nucleus. When one of the neutrons in the carbon atom decays, ejecting an electron and an antineutrino, the parent atom is no longer carbon, it has become nitrogen.
Within a neutron there are three quarks, two down quarks and one up quark. (Yes the names are weird!)
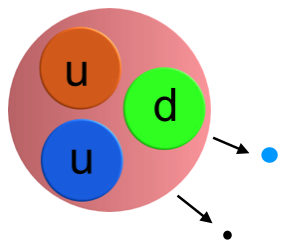
A down quark has a charge of -1/3 and an up quark a charge of +2/3 so the overall charge is zero.
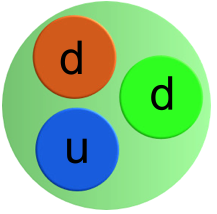
When the neutron decays one of the down quarks transforms to an up quark. The overall change in charge is from -1/3 to +2/3 so a total charge of -1 is emitted (that is an electron).
To maintain the overall lepton number an antineutrino is also emitted.
Below is the Feynman diagram of the process. Note that this is a diagram, not a graph. The Y axis is going forward in time but the X axis has no meaning.