Radioactivity and unstable atoms
Some materials are radioactive because the nucleus of each atom is unstable. These are atoms that that don’t have enough energy to bind the nucleus together. This might be due to an imbalance between the number of protons and neutrons or just because the atoms are too big. When that is the case bits are spat out of the nucleus. There are three main types of radioactivity, called alpha, beta and gamma after the first three letters of the Greek alphabet. After emitting a particle the nucleus is changed. After alpha or beta decay the atom becomes a different element because the number of protons in the nucleus has changed.
Alpha decay An alpha particle is relatively large, identical to a helium nucleus, being made up of two protons and two neutrons bound together. Alpha particles are usually emitted from one of the elements with very large atoms. As the nucleus looses two protons as well as two neutrons the process changes the original atom into a different element.
Beta decay
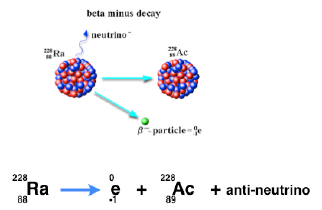 There are two kinds of beta decay: β+ and β-.
There are two kinds of beta decay: β+ and β-.
β- emission occurs by the transformation of one of the neutrons in the nucleus into a proton, an electron and an antineutrino.
β+ decays is a similar process, but involves a proton changing into a neutron, a positron and a neutrino. This is much more rare because it requires an input of energy from a rearrangement of the other particles in the nucleus.
Gamma decay
After a nucleus undergoes alpha or beta decay, it is often left in a high energy level. To reduce that gamma radiation is produced. Gamma radiation is an electromagnetic wave. It is similar to visible light but with a much shorter wavelength and far more energy.
There are more useful pages about different aspects and uses of radioactivity here:

